 Recently, Covidien, LLC, a subsidiary of Medtronic, recalled Palindrome and Mahurkar hemodialysis catheters due to a catheter hub defect. Once again, we are reminded that patient safety and supply chain are not separate parts of healthcare that should function independently of each other. Rather, their interdependence is vital to increasing patient safety and optimizing clinical outcomes; creating efficient, safe, and successful working environments; and maintaining the fiscal viability of healthcare facilities, be they large or small, private or public.
Recently, Covidien, LLC, a subsidiary of Medtronic, recalled Palindrome and Mahurkar hemodialysis catheters due to a catheter hub defect. Once again, we are reminded that patient safety and supply chain are not separate parts of healthcare that should function independently of each other. Rather, their interdependence is vital to increasing patient safety and optimizing clinical outcomes; creating efficient, safe, and successful working environments; and maintaining the fiscal viability of healthcare facilities, be they large or small, private or public.
The COVID-19 pandemic brought this simmering issue to the surface, and healthcare provider organizations are getting smarter about preparing for the unexpected and strengthening their supply chains. ECRI has long offered such advice and support, and we continue to expand and strengthen our guidance, specifically in the area of providing trustworthy, independent functional equivalent information and vendor information that is available quickly, easily, and cost-effectively—most importantly, it’s reliable information.
More than one million devices recalled
The Covidien recall includes Palindrome and Mahurkar hemodialysis catheters manufactured between June 2017 and April 2022. The associated distribution dates are June 28, 2017 to May 11, 2022. In the U.S. alone, there are 1,032,377 devices affected, and their lot information can be found in the FDA’s database.
The U.S. Food and Drug Administration identified the action as a Class I Recall. By the FDA’s definition, a Class I recall is “a situation in which there is a reasonable probability that the use of, or exposure to, a violative product will cause serious adverse health consequences or death.”
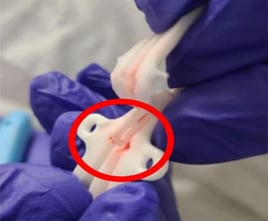
What is a Palindrome catheter used for?
The Palindrome catheter features a heparin coating and a silver ion sleeve, which are designed to reducing clot formation and allow for precise tip placement. It has been considered a premiere device, prior to the recall. Now, the manufacturer has recommended specific actions including immediately quarantining and discontinuing use for the effected lots; returning specific lots; share recall information with recipients and your own staff; and identifying the potential contamination of fluid and replace the catheter if needed.
Get alternative products information, insight, and vendor availability
With ECRI’s functional equivalents and vendor availability reports, healthcare supply chain and clinical leaders can quickly identify viable alternatives. Importantly, they also can see:
- Key performance indicators in the report. This allows you to immediately identify any differences in device functioning or appropriate procedures
- Categories so you have a deeper level of information and can see key performance indicators by category
- Match percentages: ECRI alternatives also provides key performance indicator
match percentages so you can identify differences and similarities to your existing product
Given the breadth and depth of ECRI's expertise, and insights that help you make sense of the facts, along with the vendor availability information, ECRI's functional equivalents reports are among the industry's most-trusted resources. The reports can help you create a supply chain that supports business and clinical goals, empowers decision makers, and saves time and resources.
Effectively addressing recalls is part of a resilient supply chain. Contact ECRI to learn how we can help you build a proactive, resilient supply chain.
